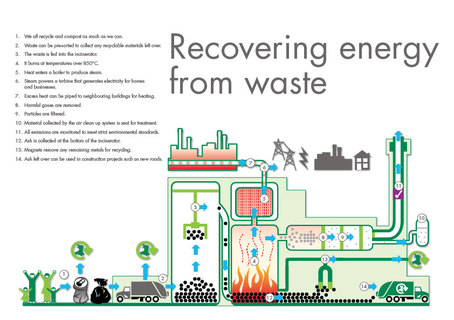
 Scientists, including those of Indian-origin, have developed a new method that can turn biomass waste into chemical products which can be used to create high-octane fuel for jets and race cars. A team of researchers from Purdue University's Center for Direct Catalytic Conversion of Biomass to Biofuels, or C3Bio, has developed a process that uses a chemical catalyst and heat to spur reactions that convert lignin into valuable chemical commodities. Lignin is a tough and highly complex molecule that gives the plant cell wall its rigid structure. "We are able to take lignin - which most biorefineries consider waste to be burned for its heat - and turn it into high-value molecules that have applications in fragrance, flavouring and high-octane jet fuels," said Mahdi Abu-Omar, Professor of Chemical Engineering and associate director of C3Bio, who led the team. "We can do this while simultaneously producing from the biomass lignin-free cellulose, which is the basis of ethanol and other liquid fuels. We do all of this in a one-step process," he said. The Purdue team developed a process that starts with untreated chipped and milled wood from sustainable poplar, eucalyptus or birch trees. A catalyst is added to initiate and speed the desired chemical reactions, but is not consumed by them and can be recycled and used again. A solvent is added to the mix to help dissolve and loosen up the materials. The mixture is contained in a pressurised reactor and heated for several hours. The process breaks up the lignin molecules and results in lignin-free cellulose and a liquid stream that contains two additional chemical products, Abu-Omar said. The liquid stream contains the solvent, which is easily evaporated and recycled, and two phenols, a class of aromatic hydrocarbon compounds used in perfumes and flavourings. A commonly used artificial vanilla flavouring is currently produced using a phenol that comes from petroleum, Abu-Omar said. The team also developed an additional process that uses another catalyst to convert the two phenol products into high-octane hydrocarbon fuel suitable for use as drop-in gasoline. The fuel produced has a research octane rating greater than 100, whereas the average gas we put into our cars has an octane rating in the eighties, Abu-Omar said. In addition to Abu-Omar, co-authors include chemical engineering graduate student Harshavardhan Choudhari, Basudeb Saha, associate research scientist in chemistry, andRakesh Agrawal, the Winthrop E Stone Distinguished Professor of Chemical Engineering. The process is described in a paper published in the Royal Society of Chemistry journal Green Chemistry. |
Ankit
|



 RSS Feed
RSS Feed
